最近一篇發表在國際學術期刊Clinical Cancer Research上的文章指出,美國啟動的抗癌登月計劃除了進行基因組學研究還應當靶向直接推動癌症發展的蛋白質。

最近一篇發表在國際學術期刊Clinical Cancer Research上的文章指出,美國啟動的抗癌登月計劃除了進行基因組學研究還應當靶向直接推動癌症發展的蛋白質。
長榮大學28日畢業典禮上,財務金融系李致緯代表接受洗腳,因他右腳裝義肢,只洗左腳,當他露出右腳的義肢,台下響起掌聲。高一罹骨癌截肢,他非但未向病魔屈服,4年來課業保持第一,還輔導成績落後學弟妹,雖然只有一隻腳,他卻引領其他人在課業上「起飛」!
穿著學士袍的23歲李致緯,拄拐杖走路,常讓人以為只是受傷,很難發現,他右腳是義肢,開朗笑容更不像生病的人。他說,或許是因經歷驟變,才明瞭過好每一天很可貴。
高一升高二時,右大腿莫名感覺痛,李致緯不以為意,一開始以為運動傷害,好幾次半夜痛到驚醒,到診所照X光無異狀,就貼膏藥、吃消炎藥,2個月後到大醫院檢查,卻被告知罹患骨癌,且右骨盆腔腫瘤長達15公分,即使切除,右腳神經仍會受損,只有截肢一途。
李致緯回想16歲乍然面對罹癌、截肢,他說,內心掙扎也怨過「為什麼是我?」但化療到手術前的9個月,他眼看一起抗癌的生命,一個個消逝,他轉念「至少我還能活」,接受截肢,醒來,右大腿以下空掉,但他知道,命保住了。
升大學時,住高雄湖內的他就近選讀長榮大學,也因不想麻煩父母而去學開車、特製車款以左腳取代右腳操作車子,每天開車通勤,由於右腳畢竟是義肢,除了洗澡、睡覺,整日穿戴不透氣護具,和同學出門頂多在室內,少了戶外共遊回憶,但大家感情很好,大學生活很精采。
他目前全心準備高普考,盼謀得穩定工作,讓父母放心;他說,選讀財金系,其實也是因生病後,媽媽為照顧自己辭掉工作,化療開銷不小,他想,若懂投資理財,日後也能分擔父母經濟壓力。
財金系主任吳宗哲說,財金系應屆畢業生約110人,李致緯4年保持系上第一名,並考取超過7張證券和期貨等相關財金證照,甚至能引導同學、學弟妹,非常難能可貴。
www.chinatimes.com/newspapers/20160529000394-260107
延申閱讀 :
抗癌鬥士劉哲瑋(小名貝貝)13年前病逝,當時才6歲的他留下許多玩具,其中最愛的湯瑪士火車陪伴他在醫院治療,他的母親昨天將這些玩具捐給彰化基督教兒童醫院,院方設立展示專區,「讓貝貝的快樂時光與更多孩童分享」。
彰基兒童醫院一樓手扶梯牆面最近出現湯瑪士火車圖像,還有展示櫥窗,醫院變得相當童趣,全球最大玩具製造商 「美泰兒」旗下擁有湯瑪士等品牌,公司得知貝貝的故事,員工昨天到兒童病房播放湯瑪士電影,也讓卡通人物「胖總管」現身,希望病童暫時忘卻病痛。
貝貝的母親陳沛淋表示,貝貝生前擁有各式各樣的玩具車,包括歐洲之星、新幹線、高鐵,尤其湯瑪士火車擺滿病床,兒子病逝後相當想念他,雖然部分玩具轉送他人,但火車系列仍保存得很好,「想讓車子有安歇的地方」。
她昇華對兒子的愛,將39組湯瑪士火車玩具捐給彰基兒童醫院,彰基兒醫過敏免疫風濕科主治醫師高峻凱則親手繪製貝貝畫像送給這名思念孩子的母親,「西瓜皮頭髮、瞇瞇眼、扁鼻子,像極了貝貝。」陳沛淋看到圖畫相當滿足。
貝貝生病時在台大醫院治療,現任彰基兒癌中心主任王士忠當時在台大學習,和貝貝感情相當好,稱醫師是「best friend」(最好的朋友)。
王士忠說,貝貝雖然生病但對生命充滿熱忱,用繪畫創作抗癌,展現超乎年齡的懂事和貼心,「與他相處感到紓壓」。貝貝的玩具如今留在兒童醫院,能與更多孩童分享他的快樂,也讓母親對他的回憶化成源源不絕的愛。

香港經濟日報記者攝。
香港前列腺基金與九龍樂善堂,過去數月在兩間位於跑馬地及荃灣的敬老康樂中心,訪問了96位年齡介乎61至92歲的男士,發現超過9成的受訪者出現不同程度的「良性前列腺增生」徵狀。
訪問以「國際前列腺症狀評分問卷」來評估他們患良性前列腺增生的嚴重程度,當中53.1%受訪者的徵狀屬「中等」、5.2%受訪者屬「嚴重」程度。
徵狀方面,85.6%受訪者表示有「夜尿」、79.8%尿流細弱無力、72.9%經常感到尿急、72.3%感小便困難等。受訪者亦表示,徵狀影響睡眠質素、日間精神、自尊心、夫婦關係及社交生活。
調查又發現,近9成的受訪者都沒有因泌尿徵狀求醫,當中約52.4%受訪者指「以為是正常老化現象,不算病徵」,更有17.7%表示因「金錢考慮」而不求診。
醫學會前會長蔡堅亦是良性前列腺增生患者,他十多年前已經出現尿頻、夜尿、尿流軟弱無力等症狀,因為擔心做手術後會有漏尿情況,一直靠藥物減輕症狀,直至兩年前某晚,尿道口劇痛「痛到在家中大叫,10級痛中達8級」,於是立即向專科醫生求診,發現出現併發症「急性尿瀦留」,要立即進行手術。蔡堅又說,手術後,徵狀只會減輕未能完全消去,例如由以往每小時要去小便,變為約每兩小時;每晚起床小便5次變為2次。
香港前列腺基金主席黃國田說,前列腺增生是男士生理必經階級,未必是病患,但不少男士基於傳統強者形象,即使發現病徵亦難以啟齒,亦不求醫,他建議,40歲以上的男士要定期接受「前列腺特異抗原(PSA)」及尿速流測試,又強調良性前列腺增生的藥物費用,每月只需約100至200元,不要因擔心藥費而不求醫。
另外,香港前列腺基金亦即場免費為100名男士檢驗「前列腺特異抗原(PSA)」水平。
延申閱讀 :
年屆花甲9成男士現前列腺增生 僅1成人求醫topick.hket.com/article/1434139/9%E6%88%90%E7%94%B7%E5%A3%AB%E6%9C%89%E8%89%AF%E6%80%A7%E5%89%8D%E5%88%97%E8%85%BA%E5%A2%9E%E7%94%9F%E5%BE%B5%E7%8B%80
逾六成受訪者出現不同程度良性前列腺增生
.jpg)
美國研究人員上個月在賓州一名49歲尿道感染婦女尿液中,發現歷來首見具有抗生素克痢黴素(colistin)抵抗力的超級病菌;克痢黴素是最後一道抗生素防線,國衛生官員說,這項驚人的發現,可能意味抗生素的功效已走到「盡頭」。
美國國防部的研究人員說,這可能是一種「真正具備廣泛藥物抵抗能力的病菌出現」的前奏;克痢黴素是用於對付特別危險的超級病菌最後防線,其中包括衛生人員稱為「惡夢病菌」的抗碳青黴烯類腸道菌(CRE)。
在某些實例中,這些超級病菌會導致半數的感染者死亡;美國疾病防治中心(CDC)曾經說,CRE已是美國最迫在眉睫的公衛威脅。
去年11月,研究人員在中國大陸與歐洲發現細菌具有代號mcr-1的抗藥性基因。研究人員並未在26日透過《抗菌藥物與化療》期刊發表報告中,說明這名婦女接受治療的結果。
CDC主任佛萊登表示,這名婦女未出國,不可能在美國以外地區感染。他說:「我們越深入探討,發現的會越多。我們可能進入後抗生素的階段。」
醫學界1959年開始以克痢黴素治療大腸桿菌、沙門氏菌、不動桿菌造成的感染,1980年代因為它會產生高度腎毒而不再用於人體,但仍然廣泛用於飼養牲口,尤其是中國大陸;由於細菌開始對其他更現代化的藥物產生抵抗性,醫院與診所再度引進克痢黴素,充當最後防線。
佛萊登表示:「我們必須設法保護抗生素,使我們與後代子孫都能持續使用。部分病人已經無藥可用。如果我們未及時採取行動,這將是抗生素的盡頭。」
堪薩斯市聖路克衛生系統的感染疾病醫師莎拉.波伊德表示:「我認為,賓州此一個案是更大的殺菌劑抗藥性問題的徵兆。」
延申閱讀 :
health.udn.com/health/story/6012/1723381
Highly resistant MCR-1 'superbug' found in US for first time
www.cidrap.umn.edu/news-perspective/2016/05/highly-resistant-mcr-1-superbug-found-us-first-time

根據英國「經濟學人智庫(Economist Intelligence Unit)」公布的臨終病人死亡品質全球性調查,台灣在亞洲18個地區中名列第三, 全球排名第六.
積極投入推動善终 / 安寧緩和醫療的新加坡全球排第十二, 香港則排名第廿二。
根據台灣衛福部統計,目前安寧緩和醫療以癌症最大宗,已有近半數癌症病人選擇安寧緩和醫療。
這項臨終病人死亡品質(Quality of Death)全球性調查,2015年受調查國家增加為80個。
延申閱讀 :
安寧缓和醫療條例 : www.tho.org.tw/xms/toc/list.php
善终 / 纾緩服務的資訊及知識 : www.ha.org.hk/haho/ho/hacp/121698c.htm
www.eiuperspectives.economist.com/healthcare/2015-quality-death-index
The UK ranks first in the 2015 Quality of Death Index, a measure of the quality of palliative care in 80 countries around the world released today by The Economist Intelligence Unit (EIU). Its ranking is due to comprehensive national policies, the extensive integration of palliative care into the National Health Service, a strong hospice movement, and deep community engagement on the issue. The UK also came top in the first Quality of Death Index, produced in 2010.
The Quality of Death Index, commissioned by the Lien Foundation, a Singaporean philanthropic organisation, is based on extensive research and interviews with over 120 palliative care experts from across the world. It shows that in general, income levels are a strong indicator of the availability and quality of palliative care, with wealthy countries clustered at the top. Australia and New Zealand take second and third place, as they did in 2010, while rich European and Asian countries dominate the top 20, along with the US in 9th place and Canada in 11th.
As expected, many developing countries are still unable to provide basic pain management due to limitations in staff and basic infrastructure. Yet some countries with lower income levels demonstrate the power of innovation and individual initiative. For example, Panama (31st) is building palliative care into its primary care services, Mongolia (28th) has seen rapid growth in hospice facilities and teaching programmes, and Uganda (35th) has made huge advances in the availability of opioid painkillers.
For the first time The EIU has also compared the supply of palliative care—as revealed in the Index—with the demand for such care. The demand analysis, based on countries’ demographic profiles and the burden of diseases for which palliative care is necessary, shows China to be among the most vulnerable from population ageing and the rising incidence of conditions such as cardiovascular disease, which accounted for one-third of all deaths in the country in 2012. Many other developing countries will also need to work hard to meet rising future need as the incidence of non-communicable disease increases and their populations grow older.
.jpg)
日期: 2016年6月3日

點解我廿二歲就有CANCER??
點解我咁靚女要甩頭髪??
唔好話有Cancer,喺塊面生咗粒瘡你都會不停問點解嘅?點解嘅?點解嘅?
醫生你唔係諗住一句基因異變就可以打發我走呀?你唔係諗住話原因有好多,我就可以安息呀?
事實係…一日入面,呀醫生要面對幾十個相同嘅case!對佢黎講已經習慣咗個個都天崩地裂咁。佢可以做嘅係同你講:你好好接受治療啦,我地會盡力幫你!
其實呢啲時間可以做倒嘅係俾自己靜下…
其實…It's okay to be not okay!
<你有病就睇醫生>
斷估你咳下唔會覺得自己有Cancer掛?
(如果會…我諗你太大壓力,都係睇醫生穩陣!)
我唔係要嚇大家!不過老實講,我最主要徵狀係咳。其他嘅徵狀唔用心加以留意,只會當係感冒影響。
事源係2013年6月尾,我本住天不怕地不怕嘅精神跟學校Study Tour去東京。回港後就隨即投入Intern 嘅Office 工作。
唔係講笑!番咗幾日工,啲同事個個輪住病,個個都咳到肺癆咁款。(我相信絕對唔係我邪住佢地呀下!)
幾日後,我係不負眾望地加入咗【咳到痴肺大家庭】。其實我都醒!咳咗幾日,加上“覺得”咳到心口有啲痛就即刻狗衝去睇醫生,話俾醫生知你地班人傳染我!
呀醫生:「你可能咳得太勁所以拉親啲肌肉姐!開啲止痛藥俾你啦。」
如是者之後覆診都得到相同回覆····· (其實又唔怪得呀醫生咁老定,鬼估倒彤姐你廢到咁咩!)個多月後,一次照鏡嘅時間竟然發現左邊鎖骨被心口腫脹的肌肉遮住。「我個性感鎖骨去咗邊呀?媽呀…」於是淆底嘅我就超級心緒不寧地去搵一個勁嘅醫生睇睇啦!(所謂勁,即係收得貴啲囉)
講解下我當時已經有嘅徵狀先:
1. 咳咳咳
2. 消失的鎖骨
3. 心口痛&背痛(唔能夠打側壓住左邊訓)
4. 一次訓覺出大汗
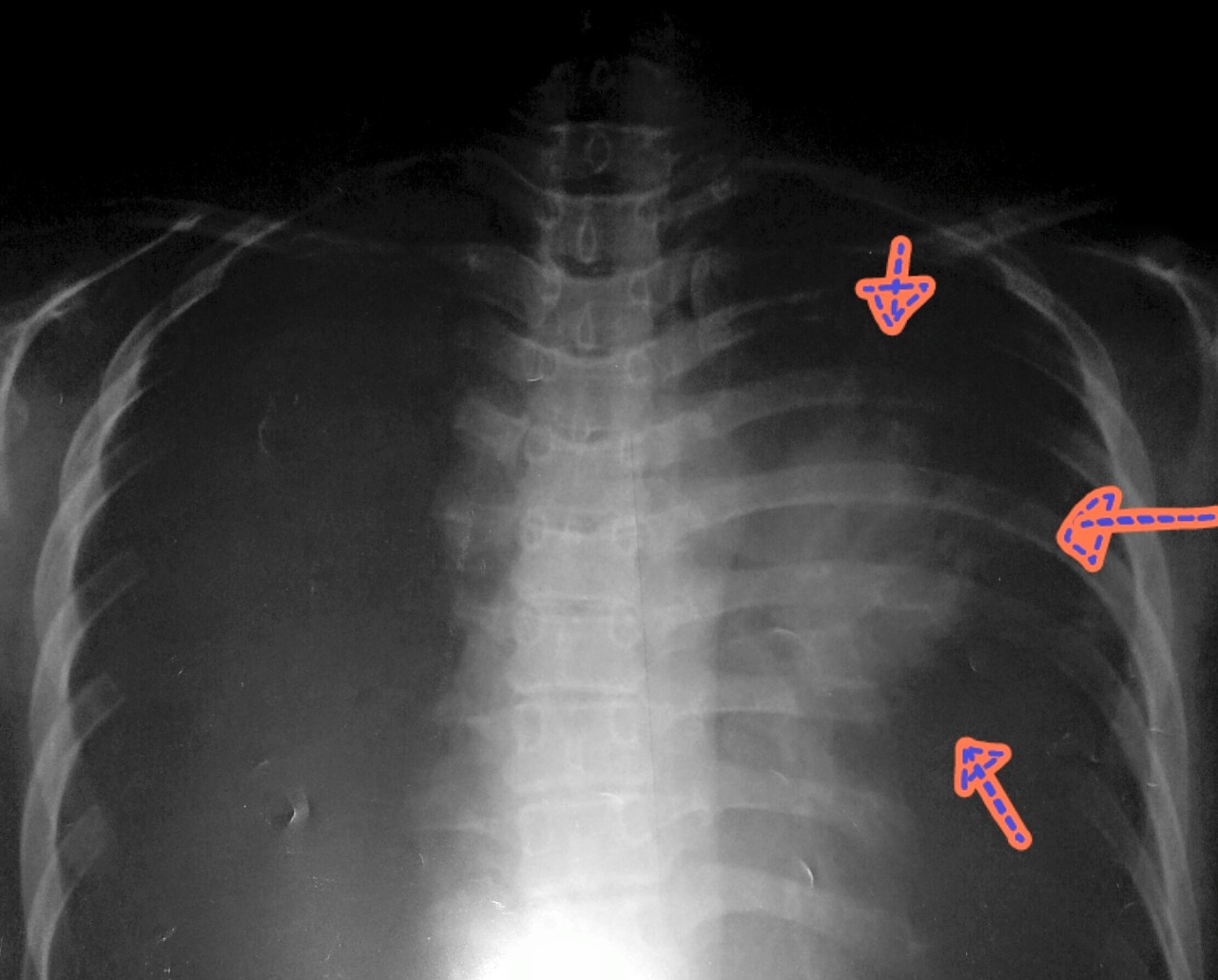
拿!人家收得貴係有原因!勁醫生摸摸我條頸後,就命令我即刻去照肺。下圖就係我當時照X-ray嘅底片。望望右邊(即我嘅左肺),見唔見倒有個橙?好記得當時我同友人拎呢張 X-ray片時,低能無腦嘅我地係指住個“橙”笑左好耐,因為我地以為係心臟又le又lo…其實惡夢已經漸漸走近身邊,但兩個傻仔仍然笑得好開心!
之後發生咩事呢??下回再分解…
P·S· 呢個橙足足有15-16cm! 其實我都唔明解,有個橙喺心口我都可以唔知…真叻豬!
彤姐話你知 : Cancer 咋嘛駛乜驚呀!
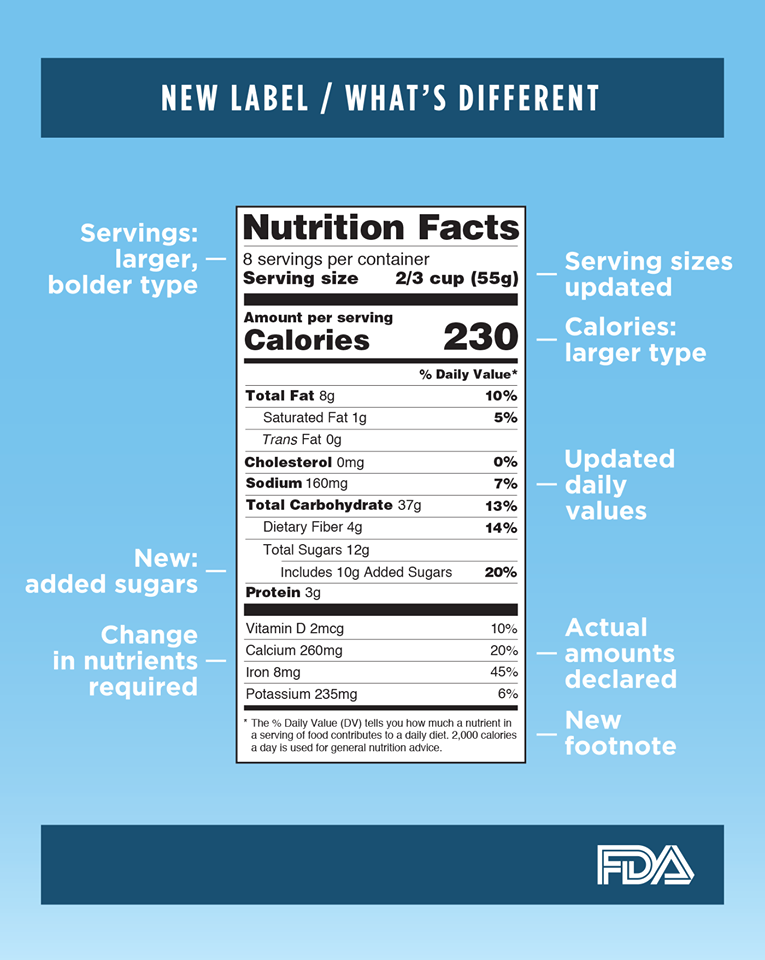
美國食品藥物管理局 ( FDA ) 於 20/5/2016 頒布包裝食品新的營養成分標籤,以反映最新的科學信息,包括飲食和慢性疾病,如肥胖和心臟疾病之間的聯繫。新標籤將使它更容易為消費者做出更好的食物選擇。