| 日期: | 2017年9月30日(星期六) |
| 時間: | 下午三時正至五時正 |
| 地點: | 香港大學百周年校園賽馬會教學樓11樓社會科學會議廳 |
| 講者: | David Kissane 教授 澳洲蒙納士大學精神病學系教授 |
| 陳智豪博士 香港中文大學社會工作學系副教授 |
|
| 胡金榮醫生 香港明愛醫院紓緩治療部副顧問醫生 |
|
| 講座流程: | 點擊這裡 |
| 講座簡介: | 點擊這裡 |
| 報名: | 點擊這裡 |
%20Rev.jpg)
| 日期: | 2017年9月30日(星期六) |
| 時間: | 下午三時正至五時正 |
| 地點: | 香港大學百周年校園賽馬會教學樓11樓社會科學會議廳 |
| 講者: | David Kissane 教授 澳洲蒙納士大學精神病學系教授 |
| 陳智豪博士 香港中文大學社會工作學系副教授 |
|
| 胡金榮醫生 香港明愛醫院紓緩治療部副顧問醫生 |
|
| 講座流程: | 點擊這裡 |
| 講座簡介: | 點擊這裡 |
| 報名: | 點擊這裡 |
%20Rev.jpg)
隨著香港人口老化,晚期病患長者人數不斷攀升,公眾對社區晚期病人護理服務的需求亦日漸增加。為此,香港賽馬會慈善信託基金於2015年撥款一億三千一百萬港元推行為期三年的「賽馬會安寧頌」計劃,協助改善社區晚期護理服務的質素,以及為相關服務的專業人員提供培訓,並舉辦公眾教育活動。

「賽馬會安寧頌」結合跨界別力量,透過不同服務模式聯繫社區及醫療系統,強化現有臨終護理服務。計劃會在社區試行五項創新服務模式,為晚期病患長者提供全面的支援,讓他們可以在充份知情下作出合適的臨終護理選擇,提升他們的生活質素。計劃合作夥伴包括:香港大學社會科學學院、香港中文大學賽馬會老年學研究所、香港老年學會、基督教靈實協會、香港復康會、聖雅各福群會,及聖公會聖匠堂長者地區中心。
項目團隊 :
培訓及教育計劃 (醫護人員)

香港中文大學賽馬會老年學研究所於2014年成立。研究所積極透過社區服務計劃、培訓及研究,為社會克服香港人口老化所帶來的挑戰作出貢獻。
地區性的安老院舍支援
香港老年學會於1986年開始由一群熱心於安老服務工作的人士發起,學會的成員來自不同的界別,包括醫療、護理、社會工作、物理治療、職業治療、心理學家及學者等。本會並於1989年成為國際老年學會之會員。本會成立的主要目標是促進香港老年學的發展和提升香港安老服務之水平。
非牟利機構創新服務

基督教靈實協會於1953成立,是一所非牟利的基督教醫療及綜合社會服務機構。過往六十多年,靈實經歷了許多不同的轉變,時至今日,靈實已發展至現今提供醫療、長者、復康和家庭服務的綜合社會服務機構,服務更遍佈整個將軍澳區,並逐漸擴展至西貢以及東九龍等其他地區。

香港復康會於 1959 年成立,是香港認可的註冊慈善團體。香港復康會具超過 50年的服務經驗,為殘疾人士、慢性病患者及長者提供各類適切而優質的服務,包括無障礙交通及旅遊、復康和持續照顧服務。
聖公會聖匠堂自1954年,便紥根於九龍城基層社區,一直關懷區內弱勢社群需要。多年來,秉承香港聖公會「非以役人,乃役於人」的服務精神,設立不同的社會服務單位,包括社區中心及長者地區中心,全力為區內不同年齡及階層人士提供全面及優質的服務,促進社會大眾的福祉。

聖雅各福群會由聖公會何明華會督及熱心人士於1949年創辦。現時,福群會在全港有58個服務點,為不同地區人士提供服務。聖雅各福群會每年服務超過四百多萬人次;服務對象包括幼兒、學童、青少年、成年人、家庭、長者及復康人士等。福群會提供多元化服務,由社區支援至院舍照顧,以配合不同人士的需要。
專業能力培訓、知識與技術轉移及成果效益評估
香港大學社會科學學院於1967年成立,此後迅速發展和不斷改革。學院設有五個學系,包括地理系、政治與公共行政學系、心理學系、社會工作及社會行政學系和社會學系;以及六個跨學科研究中心,為全球研究交流和校內外學術合作提供廣闊平台。透過「社會創新」(Social Innovation) 及「全球公民」(Global Citizenship) 的概念,我們致力促進社會進步,領導本地,區域乃至全球的發展。
項目總監
項目主研究員
 Cellectis CEO André Choulika。(圖:AFP)
Cellectis CEO André Choulika。(圖:AFP)
南方日報今 (8) 日報導,美國食品藥物管理局 (FDA) 在上個月底 (8 月 30 日) 批准了諾華使用新的癌症治療技術 CAR-T ,不過就在看似一片向好的前景下,同樣使用相關療法的法國生物製藥公司 Cellectis(CLLS-US),因為有病人接受治療 8 天後死亡,該公司被美國 FDA 要求停止試驗 CAR-T 療法 UCART123。
報導指出, Cellectis 公司在 9 月 4 日宣布 CAR-T 治療療法 被 FDA 喊停。主因為 78 歲的男性病人罹患了罕見的惡性淋巴瘤,急性漿細胞樣樹突狀細胞瘤 (BPDCN)。該病人使用過後,結果在治療的 8 天後,出現致命反應而死亡。該聲明發布後, Cellectis 股價在 9 月 5 日這天暴跌了 32%,市值一天蒸發超過 4 億美元。
 法國生物製藥公司 Cellectis 使用的 CAR-T 療法 UCART123 造成患者死亡,5 日股價暴跌 3 成,逾 4 億美元市值蒸發。(圖截自 Google)
法國生物製藥公司 Cellectis 使用的 CAR-T 療法 UCART123 造成患者死亡,5 日股價暴跌 3 成,逾 4 億美元市值蒸發。(圖截自 Google)
環球醫訊 (7) 日報導,今年 2 月, Cellectis 宣布其通用型 CAR-T 療法 UCART123 獲得美國 FDA 批准,這也是第一款獲得美國 FDA 批准進入臨床試驗的產品,也促使該公司股價扶搖直上,高點來到每股 32 美元,為 1 年來的最高點,8 月, Cellectis 宣布 UCART123 對首名 BPDCN 患者進行治療,然而卻迎來患者死亡的不幸消息。
目前 Cellectis 正與調查人員與美國 FDA 合作,希望通過修定的方案來盡速恢復試驗,而周五 Cellectis 股價目前為每股 25.51 美元,盤中漲幅 2.2%。
南方日報報導, Cellectis 不是第一家在 CAR-T 藥物試驗中導致病人死亡的公司。2016 年生物製藥公司 Juno Therapeutics 在 7 位病人死亡後暫停試驗,後來 FDA 介入改變了試驗方法,卻造成更多人死亡,該公司遭要求停止所有相關試驗。2017 年,被業內看好的 Kite 公司,在 CAR-T 項目 axicabtagene ciloleucel,也在 4 月底造成 一名患者死亡。
然而 Cellectis 的事件,波及範圍恐怕更廣,8 月 28 日,吉利德公司 (Gilead)(GILD-US) 用 119 億美元重金收購 Kite Pharma 就是看中可能很快獲批的 CAR-T 產品,直接成為諾華藥廠的最大競爭對手,原本美 FDA 預計審批該產品的期限在 2017 年 11 月 29 日,是否會因此遭到審批速度的放緩,有待觀察。
與 CAR-T 相關的製藥公司,根據科訊醫療網整理,分別有 Novartis 諾華 (NOVN-VTX)、 Kite、Juno、 Cellectis、Servier、Pfizer 輝瑞 (PFE-US)、Bellicum(BLCM-US)、 Bluebird(BLUE-US) 、Celgene 賽基 (CELG-US)
http://news.cnyes.com/news/id/3913140
FDA hits Cellectis’ off-the-shelf CAR-T program with clinical hold after first patient treated in phase 1 trial dies

The FDA has placed a clinical hold on two phase 1 trials of Cellectis’ UCART123 after learning of the death of one patient. Development of the off-the-shelf CAR-T therapy is now in limbo while Cellectis works with the FDA to redesign the protocol to mitigate the risks identified in the first weeks of the trials.
Doctors at the MD Anderson Cancer Center dosed the first blastic plasmacytoid dendritic cell neoplasm (BPDCN) patient with CD123-targeting CAR-T UCART123 on August 16. The patient died nine days later.
Initially, the 78-year-old man responded to the lowest dose of UCART123 without complication. On day five the patient suffered a grade 2 cytokine release syndrome (CRS) and grade 3 lung infection. On day eight he experienced a grade 4 capillary leak syndrome (CLS) and a CRS that, despite treatment with corticosteroids and tociluzumab, played a central role in his death the next day.
The only patient treated with UCART123 in the other phase 1 trial experienced similar, albeit less severe, reactions. That patient, a 58-year-old woman with acute myeloid leukemia (AML), suffered a grade 3 CRS and grade 4 CLS nine days after treatment with UCART123. The adverse events put the patient in intensive care but had cleared up by day 12.
The list of adverse events suffered by the patients suggests UCART123 may be affected by safety issues both general to CAR-Ts and specific to its targeting of CD123. CRS is a known and, in the case of autologous products, generally manageable side effect of CAR-Ts. The process for managing the events is well established enough for Roche to have won approval for tocilizumab—also known as Actemra—as a treatment for CAR-T-induced CRS.
It is conceivable Cellectis can prevent further patient deaths by lowering the dose of UCART123 and follow the example set by other CAR-T trials by intervening earlier and more aggressively to treat CRS.
“We think one of the key learnings from the CD19 CAR-T trials is the early administration of steroids; however, we think early administration of steroids was withheld due to potential negative impact on cell persistence. In our view, [Cellectis] may need to re-evaluate the timing of steroid administration and be more aggressive in treating CRS,” analysts at Jefferies wrote.
The way to manage the adverse events that may be tied the candidate’s targeting of CD123 is less clear.
Stemline Therapeutics’ SL-401 is the precedent in this case. Three patients in clinical trials of CD123-directed therapy SL-401 have died after experiencing severe cases of CLS, a syndrome characterized by the leaking of blood plasma through capillary walls and into the surrounding tissue. Stemline added dosing and safety parameters to the lead-in stage of the study following the first two grade 5 cases of CSL. But those precautions failed to prevent the third death.
Cellectis now needs to figure out its own precautions before it can resume enrolling the 71 BPDCN patients and 155 AML patients it plans to treat across the two phase 1 trials. The data safety monitoring board has already proposed lowering the dose of UCART123 and capping the amount of the chemotherapy cyclophosphamide patients receive during the preconditioning stage. The two patients treated to date received cyclophosphamide alongside fludarabine.
The one bright spot for Cellectis in the otherwise grim safety data is the lack of reports of graft-versus-host disease (GvHD), a complication that arises when the immune system rejects an allogeneic transplant. Cellectis’ use of T cells from donors, rather than patients themselves, means GvHD is a potential concern. Cases of the condition would scuttle Cellectis’ ambition to capture the CAR-T market from Novartis and soon-to-be Gilead unit Kite Pharma by industrializing the CAR-T production process.
Shares in Cellectis opened down about 30% in Paris.

乳癌患者在抗癌路上,最辛苦、最徬徨的莫過於化療,持續數月的治療,看著頭髮一根一根的掉落,面對無數的未知數,哪裡買假髪?我可唔可以返工?醫生可以幫到的其實不多,我們著實需要一些同路人引領我們,幫助我們渡過難關,她們便是今集的主角,落入凡間的粉紅天使⋯⋯
初初接觸粉紅天使,是在一個偶然的情況下收到她們的宣傳單張⋯⋯

「全球華人乳癌組織聯盟⋯⋯咩組織黎㗎?咁似D『亞太區』最受歡迎男歌手咁嘅,『亞』皆老街以北『太』子道西以南嗰D『亞太區』,係咪呃人㗎?」
之後放埋一邊,直到有一日,有位叫Candy 嘅小姐陪病友黎睇醫生⋯⋯
「咦,乜咁熟口面嘅?咁似我喺Facebook喺蘋果報導見過⋯⋯」

圖片來源:蘋果日報
無錯,她就是人稱關姐姐(唔係關家姐),『乳癌路上』的作者,Candy Kuan賴關裕穠。
她主動提出送出她的著作「乳癌路上」去診所給新確診乳癌的同路人,細看之下裡面還有一張單張,那一張我開始以為係呃人嘅單張。
「咁大隻蛤那隨街跳?免費陪做化療?」
原來呢個世界真係有咁大隻蛤那,仲要唔止一隻,係幾十隻。(請原諒我咁形容我們可愛的天使們,不過咁寫順暢D,絕無不敬之意)
喺Candy嘅穿針引線底下,有幸認識到「化療陪診服務」的牽頭人-Mary Wong。

過來人Mary從自身的體會,加上在乳癌組織服務多年的經驗,明白到社會正正缺乏呢方面的服務,一個離納入正規醫療機構還有很遙遠距離的服務。
她們的理念很簡單,就係攜手同行化療路,由到達醫院一刻,直到回到家中,無時無刻陪伴在側。
為免瓜田李下,服務對象暫時局限在八間公立醫院接受化療的病友。
在收到陪診服務要求後,Candy就會按住期數、年齡、所屬區份(包括接受化療的醫院及住址)等等因素進行配對,盡量安排一至兩個情況相近的天使負責照顧。在化療當天,分別會在以下地點集合,屆時天使會穿上漂亮的制服以資識別。
瑪麗醫院:S座一樓

東區醫院:入院登記處

伊利沙伯醫院:R座地下

瑪嘉烈醫院:急症室門口

聯合醫院:入院部
明愛醫院:懷明樓地下

威爾斯親王醫院:包玉剛癌症中心地下

屯門醫院:會計部地下

以上圖片由天使走訪八大醫院提供
之後天使會陪你登記、陪你交費、陪你等配藥、在漫長的等候過程陪你傾計,分享一D實用的小貼士比你,直到你落完藥陪埋你返屋企。
為咗保障病人私隱及義工安全,一般會在屋企樓下,或交通總匯(例如地鐵站、巴士站)分別。之後夜晚關姐姐會分別致電病友同天使確保一切順利。其實Mary同Candy真係好細心,佢地連義工天使的情緒也照顧到,因為始終無條件付出,同行一條過往的不愉快道路,負面情緒係可以預期的,所以下一步組織的方向就係為義工提供陪訓,將一D負面嘅野變成正面,令天使更加堅強,助人自助。
呢個服務由2017年4月開始起動,需求只會越來越大,實在需要同路人的大力支持,大家不妨在克服人生最大逆境的同時,嘗試由另一角度賦予它一種積極正面的意義。沒有你的幫助,神話係冇可能延續下去的。如果你有興趣幫手,可以打電話35958678聯絡關姐姐。
另一方面,如果朋友有這方面的需求,不妨分享粉紅天使的故事給她們,讓她們在黑暗中找到一點光。

馬國權醫生 外科專科
香港的乳癌治療資訊 http://www.breasthk.com/facing-breast-cancer/
癌症分原發性及繼發性兩大類。以肝癌為例,原發性肝癌是指始於肝臟的癌症,稱為肝細胞癌(HCC),而由身體其他部位的癌細胞轉移影響肝臟的癌症則為繼發性肝癌,例如由腸癌擴散至肝臟便可導致繼發性肝癌。原發性及繼發性肝癌雖同稱為肝癌,但治療概念卻不盡相同,因此用藥及治療方案也會有很大差異。
每年近1600人死於肝癌
癌變始於肝臟的原發性肝癌,是香港常見癌症之一,每年有逾一千八百宗新症,並導致近一千六百人死亡,男女比例約為三比一。
肝癌的發病高峰期為50至70歲,但30至50歲的壯年患者也頗常見。帶有乙型及丙型肝炎病毒的人屬高風險族群,應接受適當的治療以減低病變風險,並應定期檢查肝臟健康,以及早發現問題。
事實上,肝癌的早期病徵並不明顯甚至毫無徵兆,直至腫瘤逐漸增大時或可能出現腹部不適及悶痛,當腫瘤變大時可能會摸到硬塊、有腹水;若腫大的肝臟刺激到橫隔膜神經便可能會影響到接連右肩的神經,引發右肩疼痛,而患者亦可能有食慾不振、體重下降、噁心和容易疲倦、皮膚和眼白呈微黃、皮膚痕癢等症狀。
初期症狀不明顯難察覺
隨著腫瘤增大,肝臟機能會逐漸衰退,導致膽紅素積聚血液而引起黃疸,令眼白和皮膚呈黃色,小便也可能呈茶色、大便呈淺灰色,有時腫瘤更可堵塞膽管。
原發性肝癌若能及早發現,可以利用手術、射頻消融術等根治性治療帶來痊癒的希望,若較遲才發現便可能需要透過體內放療、藥物如標靶治療及免疫治療等等,幫助控制病情。
由此可見,對付原發性肝癌是要集中處理肝臟內的癌細胞,藥物也要選擇能針對肝癌細胞的種類。但繼發性肝癌的處理便不一樣。
肝癌源頭不同 治療有別
繼發性肝癌在臨床上十分常見,例如本港頭號癌症──腸癌在進入較晚期階段時,最先擴散影響的器官便是肝臟,因為肝臟是身體其他癌症常轉移到的器官。幾乎所有部位的腫瘤都有可能擴散到肝臟,於是引致繼發性肝癌,如腸癌、胃癌、胰臟癌、肺癌和乳癌等都是常見的病因。
由於繼發性肝癌是由其他部位的癌症引起,因此治療亦以原本的癌症為本,例如問題源於肺癌便要先控制肺部的癌症,選用的療法和藥物亦以能夠針對肺癌為重點,發自腸癌的腫瘤則要以腸癌藥物為主。一般來說,若源頭癌症控制得好,轉移到肝臟的腫瘤也會受控。
患病及早求醫方為上策
另一方面,雖然繼發性肝癌的治療以源頭癌症為主,但轉移至肝臟的癌細胞也可視乎情況而採取局部控制的措施,例如若腫瘤較小或數量不多,可以用手術或射融消融清除,提升控病的效果。
無論是原發性肝癌還是繼發性肝癌,盡早發現及接受適當的治療,不但能夠有更多的治療選擇,治療效果亦會更加理想。

臨床腫瘤專科張寬耀醫生
在本港屬於常見癌症的肝癌,經過醫學界多年努力,對不同階段的病情也能夠提供適切及有效的治療,例如較細小的腫瘤可利用手術清除,若已擴散亦可透過藥物幫助控制腫瘤生長,如標靶治療及免疫治療等等,為病人帶來選擇和希望。
•手術
手術的治療原理是切除腫瘤及附近受影響的組織。如腫瘤局限於肝臟並能夠完全割除,病人治癒的機會便很高;但若健康的肝臟過小、腫瘤過大或過於分散,又或擴散至其他部位,便可能需要考慮其他的治療方法。
•消融治療
射頻或微波消融術利用儀器將腫瘤局部組織加熱至超過攝氏60度,藉以殺死腫瘤細胞。視乎腫瘤大小及位置,消融治療可經皮膚穿刺、腹腔鏡或開腹等方式進行,療程進行時可使用超聲波監視腫瘤的消融程度。這種治療適合一些腫瘤小於
•栓塞治療
腫瘤栓塞是將供應營養給癌瘤的動脈堵塞或注射物質填塞,以減少癌瘤的血液供應。由於此治療會減少正常肝組織的血液供應,未必適合有肝炎或肝硬化的病人。
•栓塞化療術(TACE)
這是一種合併栓塞和化療的療法,可幫助控制病情及延長病人壽命。醫生會將化療藥物由動脈導管選擇性注入供應肝癌的血管,然後再注入微粒令供應肝癌的動脈被堵塞,鎖住藥物在腫瘤位置之餘,亦令養份無法送達腫瘤,腫瘤可因此縮小或停止生長。適用於腫瘤已擴散至兩葉肝臟但還未轉移到其他器官的病人,又或腫瘤只局限於肝的一邊但因肝功能差而無法接受手術的病人。
•放射線治療(俗稱電療)(Radiation Therapy)
放射治療是利用高能量放射線殺死或收縮癌細胞的療法,從而令肝腫瘤縮細或舒緩病徵。
•化療(Chemotherapy)
利用藥物殺死體內癌細胞,一般透過靜脈或口服輸送藥物到體內,惟化療藥物對治療肝癌一般作用不大。
•標靶治療
透過阻截腫瘤細胞生長的傳訊路徑,從而制止腫瘤不正常的生長,例如有些藥物可以針對性阻斷癌細胞生長訊號的傳遞,亦有些藥物可以阻截新血管的形成,藉以減少腫瘤的營養供應。此類治療適合一些無法接受手術或消融術等晚期肝癌病人。現在有數種新的標靶治療藥物正進行臨床研究,將來可望提高肝癌治療效果。
•免疫治療
免疫治療可以重啟身體的抗癌機制,利用自身免疫系統殺死癌細胞,部分免疫治療藥物已經投入臨床使用。
•肝臟移植
對於肝功能差但癌細胞未擴散的肝癌患者而言,肝臟移植是治癒的希望,因為過去研究顯示,符合條件的肝癌病人接受移植手術後五年的存活率可高達70%,但等待到合適肝臟捐贈者並不容易。

臨床腫瘤專科張寬耀醫生
http://www.hkioc.com.hk/tc/article/3048
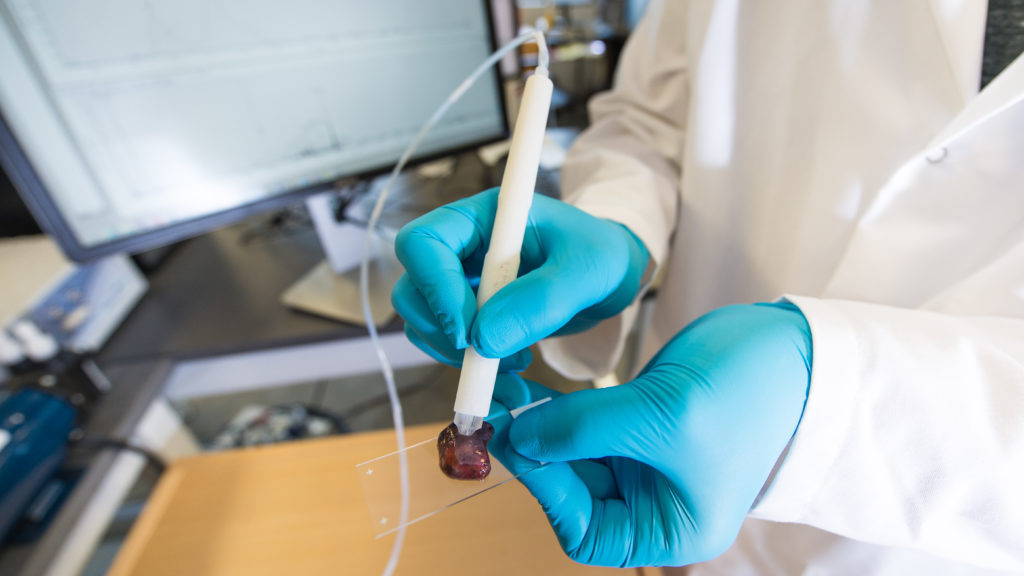
(中央社華盛頓6日綜合外電報導)癌症患者動手術時最擔心的莫過於外科醫師未將腫瘤清除乾淨,專家現在已經研發出一種新型筆狀工具,能將這樣的憂慮一掃而空。
法新社報導,美國德州大學奧斯汀分校(University of Texas at Austin)科學家和工程師研發的這項工具稱為「質譜筆」(MasSpec Pen,暫譯),能讓移除腫瘤的外科醫師在幾秒內就能偵測出組織裡的癌細胞,讓醫師立刻知道自己是否已經發現且移除所有癌細胞。
目前要花上好幾天時間才能確定外科醫師是否切除所有癌細胞;如果有所遺漏,可能會導致患者癌症復發,或至少必須再動一次手術來將病變細胞清乾淨。
美聯社引述研究主筆、德州大學奧斯汀分校化學副教授厄伯林(Livia Eberlin)表示,每個人最大夢魘,莫過於動刀後仍有癌細胞殘留的可能性,「透過提供即時分子資料,我們真的可以提高精準度」。
「科學轉譯醫學」(Science Translational Medicine)今天刊登的研究指出,這種筆在手術中只需10秒左右的時間就能辨識出癌組織。
手術期間用質譜筆接觸組織,輕輕引出小分子,再透過筆尖探針內建的小型質譜儀來進行分析。它能透過一種稱為代謝物(metabolites)的獨特分子來辨識癌細胞。
研究說:「每種癌症都會產生一組獨特的代謝物和其他作用有如指紋的生物標記。」
質譜筆能在幾秒內辨識出殘餘的癌細胞,連接質譜筆的監視器會顯示細胞為「正常」或是「癌」。這讓外科醫師得以繼續清除癌細胞,直到幾乎確定已經清除所有癌細胞為止;對於哪個組織要留在患者體內,醫師也能做出更好的判斷。
根據這項研究,自253名人類癌症患者身上移除的組織檢測顯示,這種新型工具的「準確率超過96%」。
http://www.cna.com.tw/news/ahel/201709070184-1.aspx
When it comes to treating cancer, surgeons want to get rid of as much cancerous tissue as possible during tumor removal. Now a new technology—the size of a pen—is attempting to make that easier by distinguishing between tumors and healthy tissue in just 10 seconds.
The MasSpec Pen is a real-time diagnostic tool created by researchers at the University of Texas at Austin. In a new study published Wednesday in the journal Science Translational Medicine, the researchers report that their handheld device (which is not yet FDA-approved) uses tiny droplets of water to analyze human tissue samples for cancer with 96% accuracy.
“It’s a gentle, simple chemical process,” says study author Livia Schiavinato Eberlin , an assistant professor of chemistry at UT Austin. “It’s highly specific and highly sensitive. The fact that it’s non-destructive brings a new approach to cancer diagnosis.”
MORE: Researchers Find a Way to Light Up Cancer Cells
Getting rid of all cancerous tissue while also preventing any harm to healthy tissue is a delicate process. When operating on a woman with breast cancer, for example, a doctor needs to remove the tumor and other affected tissues while maintaining the rest of the breast. Currently there are other tools available to surgeons for tissue diagnosis, but many use gases or solvents that can be harmful for the human body. In 2016, researchers in Massachusetts reportedthat they developed a probe that can find and light up cancer cells, making them easier for surgeons to see. But other methods currently available to surgeons today are slower than the MasSpec Pen, the study authors say, in some cases by 30 minutes or more.
Human cells produce a variety of small molecules, and cancer creates a unique set of them that can be used for pattern identification. The MasSpec Pen produces a small drop of water that extracts molecules from a person’s cells during surgery. Through machine learning, the MasSpec Pen is able to determine what molecular fingerprint is normal and what is cancer, Eberlin says.
In the study, the researchers tested 253 human tissue samples from lung, ovary, thyroid and breast cancer tumors and compared them to samples of healthy tissues. The device was 96% accurate at identifying cancerous tissues. The researchers also tested the MasSpec Pen in live mice with tumors and found that the device was able to identify the presence of cancer without harming healthy surrounding tissues. The device can also identify different subtypes of lung and thyroid cancer, and the team hopes to make it more specified for other types of cancer, too.
The researchers say they need to continue validating their work and that they plan to start clinical testing in humans in 2018. Until then, it’s unclear how exactly the device will work when integrated into surgery. While the pen-sized device that the surgeon would use is small, the device is connected to a large mass spectrometer, which helps the process of analyzing individual molecules . That large machine would need to be wheeled in and out of a surgery room for each procedure. The pen is disposable, so surgeons would replace it with each surgery.
“This is a good example of a tool that empowers our transition to precision medicine where the treatment can be done with much higher levels of confidence,” says study author Thomas Milner, professor of biomedical engineering in UT Austin's Cockrell School of Engineering . “Treatment can be planned and given where the outcomes are known. This is one tool along that path.”
圖片來源:SHERBROOKE CONNECTIVITY IMAGING LAB/SCIENCE PHOTO LIBRARY
寨卡病毒會導致嬰兒出生後存在嚴重腦損傷,但人們或可利用這種病毒抵抗成年人腦瘤。
寨卡病毒約在4年前從波利尼西亞到達南美洲,它在懷孕女性中尤為危險。它會導致頭小畸形(異常的小頭癥),並與母親懷孕時感染病毒的嬰兒出現神經問題存在關聯,此外還會導致更高的流產率。
寨卡病毒之所以如此,是因為它與大多數微生物不同,會從血液進入大腦,並在那裏感染及殺死幹細胞,對發育大腦造成嚴重負面影響。
但寨卡病毒影響大腦幹細胞的能力已被證實或對抵抗致命腦癌具有幫助,很多腦癌是由於幹細胞變異造成的。
美國加州大學聖疊戈分校的Jeremy Rich和團隊通過惡性膠質瘤(最常見的腦癌)測試了寨卡病毒。惡性膠質瘤是最難治愈的癌癥之一,即便在經過手術和其他治療之後,它通常還會在一年內導致病人死亡。
該團隊發現,將生長在培養皿中的惡性膠質瘤的樣本暴露給寨卡病毒之後,會損傷癌癥幹細胞。通常這些幹細胞會導致病人死亡,因為它們對所有可獲得的療法具有抵抗力。
當該團隊在未罹患癌癥的成年人的普通腦細胞上驗證寨卡病毒後,他們發現該病毒並不會影響組織,這或許可以解釋為什麼寨卡病毒鮮少在成年人中致病。
接下來,該團隊在植入惡性膠質瘤的小鼠體內驗證了該病毒。通常,這一類老鼠會在1個月內死亡,然而那些註射了寨卡病毒的小鼠生命會更長,9只小鼠中有4只小鼠在兩個月後依然存活。
Rich表示,目前尚不清楚這種機制在人體中會如何轉化,因為該疾病對小鼠的影響不同於人類。
研究人員並未計劃在患有腦癌的人中間驗證寨卡病毒,因為他們擔心它會被傳播給孕婦:美國一些區域發現了攜帶寨卡病毒的蚊蟲,該病毒可以通過性傳播。與此相對,該團隊計劃了解是否可以對該病毒進行基因編輯,使其變得更加安全,同時在研究將其作為腦癌的潛在療法。
 GETTY IMAGES
GETTY IMAGESA harmful virus that can cause devastating brain damage in babies could offer up a surprising new treatment for adult brain cancer, according to US scientists.
Until now, Zika has been seen only as a global health threat – not a remedy.
But latest research shows the virus can selectively infect and kill hard-to-treat cancerous cells in adult brains.
Zika injections shrank aggressive tumours in fully grown mice, yet left other brain cells unscathed.
Human trials are still a way off, but experts believe Zika virus could potentially be injected into the brain at the same time as surgery to remove life-threatening tumours, the Journal of Experimental Medicine reports.
The Zika treatment appears to work on human cell samples in the lab.
 GETTY IMAGES
GETTY IMAGESThere are many different types of brain cancer. Glioblastomas are the most common in adults and one of the trickiest to treat.
They are fast growing and diffuse, meaning they spread through the brain, making it difficult to see where the tumour ends and the healthy tissue begins.
Radiotherapy, chemotherapy and surgery may not be enough to remove these invasive cancers.
But the latest research, in living mice and donated human brain tissue samples, shows Zika therapy can kill cells that tend to be resistant to current treatments.
It is thought that these glioblastoma stem cells continue to grow and divide, producing new tumour cells even after aggressive medical treatment.
Different, healthy stem cells are found in abundance in baby brains, which probably explains why regular Zika can be so damaging to infants, say the researchers.
Adult brains, however, have very few stem cells. This means Zika treatment should destroy only the cancer-causing brain stem cells without causing much collateral damage.
As an extra safety precaution, the team, from Washington University School of Medicine and the University of California San Diego School of Medicine, have already begun modifying the virus to make it more tame than regular Zika.
Researcher Dr Michael Diamond said: "Once we add a few more changes, I think it's going to be impossible for the virus to overcome them and cause disease.
"It looks like there's a silver lining to Zika. This virus that targets cells that are very important for brain growth in babies, we could use that now to target growing tumours."
He hopes to begin human trials within 18 months.
Using viruses to fight cancer is not a new idea, but using Zika as the weapon of choice is.
UK scientists at the University of Cambridge are beginning similar trials with Zika.
Dr Catherine Pickworth, from Cancer Research UK, said: "This promising research shows that a modified version of the Zika virus can attack brain tumour cells in the lab.
"This could one day lead to new treatments for this particularly hard to treat type of cancer."
 REUTERS
REUTERS
香港中文大學(中大)計算機科學與工程學系教授王平安教授及其研究團隊,最近成功應用人工智能影像識別技術,通過深度學習(Deep Learning)系統判讀電腦斷層掃瞄(CT)及病理組織切片等醫學影像,並針對香港兩大高危疾病──肺癌及乳腺癌的影像進行研究。結果顯示,利用人工智能判讀該兩種癌症的醫學影像,準確率分別高達91%及99%,識別過程只需30秒至10分鐘,可見此技術能大幅提升臨床診斷的效率,並降低誤診率。預計在未來一至兩年,這種自動化檢測技術將可於本地醫療界廣泛應用。
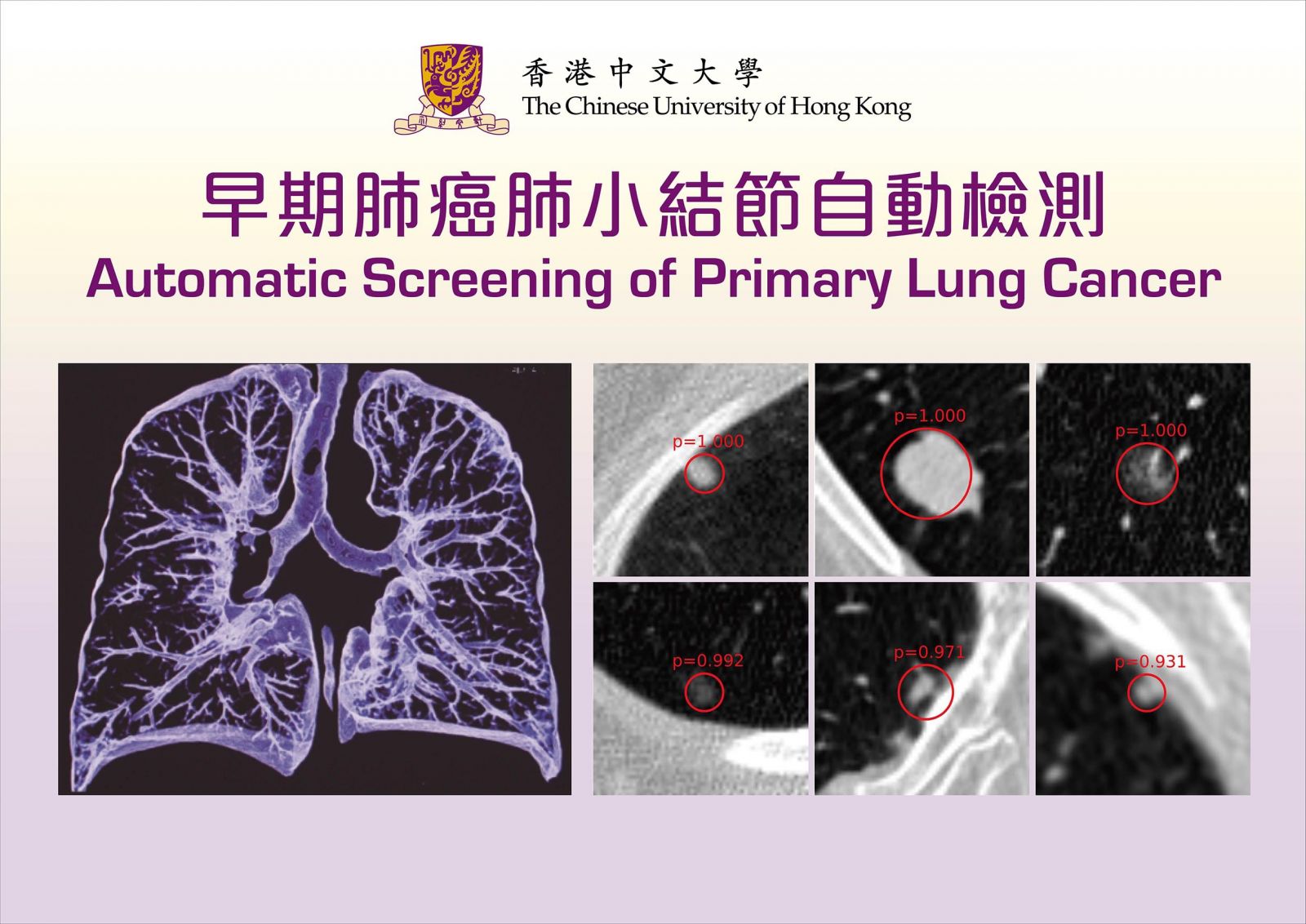
早期肺癌肺小結節自動檢測
通過深層神經網絡自動檢測肺小結節
肺癌是本港頭號致命癌症,早期肺癌多以肺小結節(small pulmonary nodule)的形式出現,即在肺部影像出現細小的團狀陰影。現時,醫生主要通過胸腔CT圖像去檢查是否存在肺小結節,然而,每次檢查都可產生多達數百張斷層掃描圖像,單靠肉眼判斷,往往耗費大量時間精力,準確度或會因醫生的經驗及精力差 異而有所影響。王平安教授及其團隊採用深度學習技術判讀CT掃描圖像,僅需30秒,就能自動識別出可能出現肺小結節的位置,準確度高達九成。一般情況下,每次CT檢查會產生數百張二維切面圖,如逐一以肉眼觀察,每幅花3秒,耗時至少5分鐘。
中大研究團隊早於五年前展開相關實驗,技術走在國際前沿,現今測試效果已取得醫學界的正面回響。王平安教授相信此技術將於未來一至兩年內被廣泛應用,他表示:「深度學習透過先進的方法,提升技術的敏感度,剔除疑似及雜訊的誤報(假陽性),解決了用肉眼檢測影像所遇到的最大挑戰。」他透露,團隊將聯同北京幾所醫院合作開發相關產品,以優化技術,及早識別肺結節病變,為肺癌的早期診斷和治療提供可靠的依據。
自動檢測組織病理學圖像中的乳腺癌淋巴結轉移
自1990年以來,本港乳腺癌新增個案持續上升。乳腺癌是香港女性最常見的癌症,在常見癌症中排名第三。醫生一般要通過乳房X光造影或MR掃瞄,檢測硬塊位置:在檢測淋巴結轉移時,醫生須切取一小塊活組織為樣本,在顯微鏡下檢查淋巴結有否轉移,以及腫瘤是良性還是惡性。一幅數碼活組織全切片圖像的解像度非常高,檔案大小可達1GB(Gigabyte),相當於一部90分鐘高清電影的儲存容量,檢測過程極之費時費力。
中大研究團隊開發了一種嶄新的深層疊卷積神經網絡,分階段處理乳腺癌的切片圖像。首先使用改良版的全卷積網絡(Fully Convolutional Network)——一種對圖像進行較粗略但保持高靈敏度的快速預測模型,重構出更加精密而準確的預測結果,最後定位並挑選出含有淋巴結轉移的圖像。整個自動化檢測過程只需約5至10分鐘,如單靠肉眼查看則病理醫生往往要花費15至30分鐘。準繩度方面,對比資深病理醫生人工檢測的結果,自動化檢測的準確度高出2%,達到98.75%,對乳腺癌的臨床診斷極具參考價值。
人工智能深度學習的優點,是能容納大規模的參數,隨著數據之累積,精準度能夠不斷提升;應用於醫學上的自動篩查及檢測,有如一個永不疲勞的醫護助手,協助醫生快速識別病源,及時制定適切的治療方案,對症下藥。
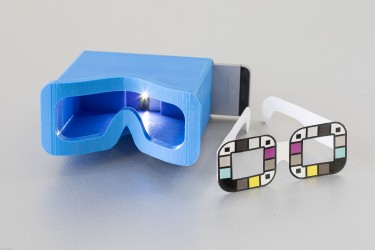
胰臟癌是死亡率最高的癌症之一,少於一成病人能活多過5年,而且病徵不明顯。美國華盛頓大學的研究人員研發了一個手機應用程式,透過分析自拍照中眼白的膽紅素水平,從而得知患胰臟癌的機率。
英國《每日郵報》報道,眼白變黃是胰臟癌的其中一個病徵,那是因為腫瘤影響體內膽紅素上升,但如果看到眼睛變黃,通常已經是癌症後期。
這個名叫BiliScreen的應用程式,能夠分析自拍者眼睛的照片,探測眼白的膽紅素水平。程式在首次的臨床測試中成功率達八成七,同時較傳統的驗血方法更方便、便宜。
程式仍需作進一步測試,希望能於九月中推出。研究人員希望能幫助不幸患上胰臟癌的人及早發現,把握時間接受治療,增加生存機會。

BiliScreen is a new smartphone app that is designed to screen for pancreatic cancer by having users snap a selfie. It’s shown here with a 3-D printed box that helps control lighting conditions to detect signs of jaundice in a person’s eye.Dennis Wise/University of Washington
Pancreatic cancer has one of the worst prognoses — with a five-year survival rate of 9 percent — in part because there are no telltale symptoms or non-invasive screening tools to catch a tumor before it spreads.
Now, University of Washington researchers are developing an app that could allow people to easily screen for pancreatic cancer and other diseases — by snapping a smartphone selfie.
BiliScreen uses a smartphone camera, computer vision algorithms and machine learning tools to detect increased bilirubin levels in a person’s sclera, or the white part of the eye. The app is described in a paper to be presented Sept. 13 at Ubicomp 2017, the Association for Computing Machinery’s International Joint Conference on Pervasive and Ubiquitous Computing.
One of the earliest symptoms of pancreatic cancer, as well as other diseases, is jaundice, a yellow discoloration of the skin and eyes caused by a buildup of bilirubin in the blood. The ability to detect signs of jaundice when bilirubin levels are minimally elevated — but before they’re visible to the naked eye — could enable an entirely new screening program for at-risk individuals.
In an initial clinical study of 70 people, the BiliScreen app — used in conjunction with a 3-D printed box that controls the eye’s exposure to light — correctly identified cases of concern 89.7 percent of the time, compared to the blood test currently used.
“The problem with pancreatic cancer is that by the time you’re symptomatic, it’s frequently too late,” said lead author Alex Mariakakis, a doctoral student at the Paul G. Allen School of Computer Science & Engineering. “The hope is that if people can do this simple test once a month — in the privacy of their own homes — some might catch the disease early enough to undergo treatment that could save their lives.”
BiliScreen builds on earlier work from the UW’s Ubiquitous Computing Lab, which previously developed BiliCam, a smartphone app that screens for newborn jaundice by taking a picture of a baby’s skin. A recent study in the journal Pediatrics showed BiliCam provided accurate estimates of bilirubin levels in 530 infants.
In collaboration with UW Medicine doctors, the UbiComp lab specializes in using cameras, microphones and other components of common consumer devices — such as smartphones and tablets — to screen for disease.

BiliScreen provides estimates of bilirubin levels in a person’s blood. Elevated levels can be an early warning sign for pancreatic cancer, hepatitis and other diseases.Dennis Wise/University of Washington
The blood test that doctors currently use to measure bilirubin levels — which is typically not administered to adults unless there is reason for concern — requires access to a health care professional and is inconvenient for frequent screening. BiliScreen is designed to be an easy-to-use, non-invasive tool that could help determine whether someone ought to consult a doctor for further testing. Beyond diagnosis, BiliScreen could also potentially ease the burden on patients with pancreatic cancer who require frequent bilirubin monitoring.
In adults, the whites of the eyes are more sensitive than skin to changes in bilirubin levels, which can be an early warning sign for pancreatic cancer, hepatitis or the generally harmless Gilbert’s syndrome. Unlike skin color, changes in the sclera are more consistent across all races and ethnicities.
Yet by the time people notice the yellowish discoloration in the sclera, bilirubin levels are already well past cause for concern. The UW team wondered if computer vision and machine learning tools could detect those color changes in the eye before humans can see them.
“The eyes are a really interesting gateway into the body — tears can tell you how much glucose you have, sclera can tell you how much bilirubin is in your blood,” said senior author Shwetak Patel, the Washington Research Foundation Entrepreneurship Endowed Professor in Computer Science & Engineering and Electrical Engineering. “Our question was: Could we capture some of these changes that might lead to earlier detection with a selfie?”
BiliScreen uses a smartphone’s built-in camera and flash to collect pictures of a person’s eye as they snap a selfie. The team developed a computer vision system to automatically and effectively isolate the white parts of the eye, which is a valuable tool for medical diagnostics. The app then calculates the color information from the sclera — based on the wavelengths of light that are being reflected and absorbed — and correlates it with bilirubin levels using machine learning algorithms.

The UW team tested two different accessories for BiliScreen: a 3-D printed box to control lighting conditions and glasses that help the app calibrate colors. The goal is to remove the need for additional accessories, potentially by mining data from facial pictures.Dennis Wise/University of Washington
To account for different lighting conditions, the team tested BiliScreen with two different accessories: paper glasses printed with colored squares to help calibrate color and a 3-D printed box that blocks out ambient lighting. Using the app with the box accessory — reminiscent of a Google Cardboard headset — led to slightly better results.
Next steps for the research team include testing the app on a wider range of people at risk for jaundice and underlying conditions, as well as continuing to make usability improvements — including removing the need for accessories like the box and glasses.
“This relatively small initial study shows the technology has promise,” said co-author Dr. Jim Taylor, a professor in the UW Medicine Department of Pediatrics whose father died of pancreatic cancer at age 70.
“Pancreatic cancer is a terrible disease with no effective screening right now,” Taylor said. “Our goal is to have more people who are unfortunate enough to get pancreatic cancer to be fortunate enough to catch it in time to have surgery that gives them a better chance of survival.”
Co-authors include Allen School undergraduate student Megan A. Banks, research study coordinator Lauren Phillipi and assistant professor of medicine Lei Yu.
The research was funded by the National Science Foundation, the Coulter Foundation and endowment funds from the Washington Research Foundation.
For more information, contact the research team at [email protected] or Mariakakis at [email protected].