科學家使用改良的沙門氏菌將抗癌藥物顆粒夾帶到腫瘤中
稿源:cnBeta.COM
發明有效的抗癌藥物是一回事,如何將它們準確送到體內腫瘤部位是另一回事。由一個高度進化的包含免疫系統保護的複雜生物體進行導航,將這些粒子一起放在腫瘤裏是一個具有挑戰性的任務,科學家們正在從各個角度繼續解決這個問題。佛吉尼亞理工大學開發的一種頗有前景的新方法依賴於沙門氏菌感染的滲透性,他們發現這種方法可以用作將抗癌納米粒子夾帶到腫瘤中的載體。
這種新技術源於身體對外來細胞的反應,如沙門氏菌和其他類型的感染所帶來的威脅。當面對這種細菌時,身體的免疫系統會發揮作用來對抗它,這可能會為交火中的癌細胞帶來壞消息。這構成了免疫療法領域的一部分,科學家正在探索增強身體免疫系統以克服癌症的方法,並不斷取得進步。
Virgina Tech團隊想知道,除了觸發身體的免疫系統外,沙門氏菌還可以增強,使抗癌藥物更有效地運送到腫瘤部位。六年前佛吉尼亞理工大學機械工程師Bahareh Behkam首次提出這個想法,這並不是一條通向成功的簡單途徑,但經過大量的修修補補後,她和她的團隊現在已經取得了一些非常有希望的成果。
他們將其命名為改良細菌的自主藥物傳遞系統,又名NanoBEADS。它由聚乳酸-乙醇酸共聚物製成的納米顆粒組成,用於攜帶抗癌藥物,化學附著於減毒細菌菌株Salmonella enterica serovar Typhimurium VNP20009。
這是一種弱化的沙門氏菌,雖然仍可引起免疫反應,但沒有沙門氏菌感染帶來的有害影響,其已在一期臨床試驗中進行了大力研究。
“沙門氏菌作為病原體的工作是穿透組織,”Behkam說。 “我們的想法是,如果細菌在組織中移動得如此擅長,那麼如何將納米醫學與細菌結合起來,將藥物傳遞得遠遠超過它自身被動擴散的範圍?”
該團隊通過將納米BEADS納入實驗室培養的腫瘤中,探索納米BEADS的滲透能力,與常規擴散納米粒子相比,納米粒子滲透和分佈的發現率提高了80倍。不滿足於此,研究人員向患有乳腺癌的小鼠施用NanoBEAD,併發現與被動遞送方法相比,它們極大地改善了實體瘤中納米顆粒的保留達100倍。
“最值得注意的是,沙門氏菌本身有助於使腫瘤中的顆粒濃度保持高達100倍,這表明它將是一種有效的運載工具,”該研究的共同作者Coy Allen說。
該團隊的研究成果發表在“高級科學”雜誌上。

文章來自: cnBeta (中)
Researchers create a bacteria-based drug delivery system that outperforms conventional methods
December 20, 2018, Virginia Tech
 Rick Davis (left), professor of chemical engineering; Bahareh Behkam (middle), associate professor of mechanical engineering; and Coy Allen (right), assistant professor of biomedical sciences and pathobiology in the Virginia-Maryland College of Veterinary Medicine. All three are affiliated with Virginia Tech’s Macromolecules Innovation Institute and have teamed up on developing their new drug delivery system called NanoBEADS Credit: Virginia Tech
Rick Davis (left), professor of chemical engineering; Bahareh Behkam (middle), associate professor of mechanical engineering; and Coy Allen (right), assistant professor of biomedical sciences and pathobiology in the Virginia-Maryland College of Veterinary Medicine. All three are affiliated with Virginia Tech’s Macromolecules Innovation Institute and have teamed up on developing their new drug delivery system called NanoBEADS Credit: Virginia Tech
An interdisciplinary team of three Virginia Tech faculty members affiliated with the Macromolecules Innovation Institute has created a drug delivery system that could radically expand cancer treatment options.
The conventional cancer treatment method of injecting nanoparticle drugs into the bloodstream results in low efficacy. Due to the complexities of the human body, very few of those nanoparticles actually reach the cancer site, and once there, there’s limited delivery across the cancer tissue.
The new system created at Virginia Tech is known as Nanoscale Bacteria-Enabled Autonomous Drug Delivery System (NanoBEADS). Researchers have developed a process to chemically attach nanoparticles of anti-cancer drugs onto attenuated bacteria cells, which they have shown to be more effective than the passive delivery of injections at reaching cancer sites.
NanoBEADS has produced results in both in vitro (in tumor spheroids) and in vivo (in living mice) models showing up to 100-fold improvements in the distribution and retention of nanoparticles in cancerous tissues.
This is a product of the five-year National Science Foundation CAREER Award of Bahareh Behkam, associate professor of mechanical engineering. Collaborators on this interdisciplinary team are Rick Davis, professor of chemical engineering, and Coy Allen, assistant professor of biomedical sciences and pathobiology in the Virginia-Maryland College of Veterinary Medicine.
“You can make the most amazing drugs, but if you cannot deliver it where it needs to go, it cannot be very effective,” Behkam said. “By improving the delivery, you can enhance efficacy.”
This work, which combines expertise in mechanical engineering, biomedical engineering, chemical engineering, and veterinary medicine, was recently detailed in Advanced Science.
Using salmonella for good
Humans have noticed, even as far back as Ancient Egypt, that cancer went into remission if the patient also contracted an infection like salmonella. Neither are ideal, but humans can treat salmonella infections more effectively than cancer.
In modern times, Allen said the idea of treating cancer with infections traces back to the late 1800s and has evolved into immunotherapy, in which doctors try to activate the immune system to attack cancerous cells.
Of course, salmonella is harmful to humans, but a weakened version could in theory provide the benefits of immunotherapy without the harmful effects of salmonella infection. The concept is similar to humans receiving a weakened flu virus in a vaccine to build immunity.
Over six years ago, Behkam came up with the idea of augmenting bacterial immunotherapy to also attack cancer with conventional anti-cancer drugs. The problem was the passive delivery of anti-cancer drugs doesn’t work very well.
Given her background in bio-hybrid microrobotics, she wanted to use salmonella bacteria as autonomous vehicles to transport the medicine, in nanoparticle form, directly to the cancer site.
The work began with Behkam’s first doctoral student, Mahama Aziz Traore, constructing the first generation of NanoBEADS by assembling tens of polystyrene nanoparticles onto E. coli bacteria. After thoroughly studying the dynamics and control aspects of the NanoBEADS systems for a few years, Behkam brought Davis into the project because he had experience creating polymer nanoparticles for drug delivery.
“She mentioned this radically different approach for delivering drugs and nanoparticles,” Davis said. “I walked away from the conversation thinking, ‘Man, if this thing could work, it would be fantastic.'”
Behkam chose this particular bacterial strain, Salmonella enterica serovar Typhimurium VNP20009, because it has been thoroughly studied and successfully tested in a phase one clinical trial.
“Its (salmonella’s) job as a pathogen is to penetrate through the tissue,” Behkam said. “What we thought is if bacteria are so good at moving through the tissue, how about coupling nanomedicine with the bacterium to carry that medicine much farther than it’d passively diffuse on its own?”
graphical video showing how nanoparticles are attached to salmonella bacteria cells which move between cells to reach tumors
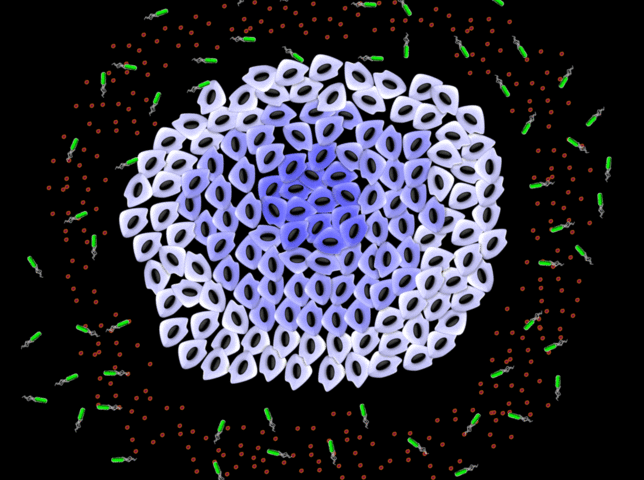 NanoBEADS agents are constructed by conjugating poly(lactic‐co‐glycolic acid) nanoparticles with tumor‐targeting Salmonella typhimurium. NanoBEADS enhance retention and distribution of nanoparticles in solid tumors by up to a remarkable ≈100‐fold, through intercellular (between cells) self‐replication and translocation. This transport enhancement is achieved autonomously, without the need for any externally applied driving force or control input. Credit: Virginia Tech
NanoBEADS agents are constructed by conjugating poly(lactic‐co‐glycolic acid) nanoparticles with tumor‐targeting Salmonella typhimurium. NanoBEADS enhance retention and distribution of nanoparticles in solid tumors by up to a remarkable ≈100‐fold, through intercellular (between cells) self‐replication and translocation. This transport enhancement is achieved autonomously, without the need for any externally applied driving force or control input. Credit: Virginia Tech
Description of graphical element: NanoBEADS agents are constructed by conjugating poly(lactic?co?glycolic acid) nanoparticles with tumor?targeting Salmonella typhimurium. NanoBEADS enhance retention and distribution of nanoparticles in solid tumors by up to a remarkable ?100?fold, through intercellular (between cells) self?replication and translocation. This transport enhancement is achieved autonomously, without the need for any externally applied driving force or control input.
Trial and error
Although Behkam had a vision for the new drug delivery system, it took several years for it to become reality.
“The process of creating nanoparticles and then attaching them to bacteria in a robust and repeatable manner was challenging, but add on top of that ensuring the bacteria stay alive, discovering the mechanism of bacteria transport in cancerous tissue, and devising ways to quantitatively describe the effectiveness of NanoBEADS, and this was a difficult project,” Davis said.
SeungBeum Suh, Behkam’s former Ph.D. student, and Amy Jo, Davis’ former Ph.D. student, worked together on attaching nanoparticles while keeping the bacteria alive. It wasn’t until their fourth attempt that they started finding success.
“We collaborated to make these particles, and we attached them to the bacteria,” Behkam said. “Then the question was what is the mechanism of their translocation in the tumor? How far do they go into the tumor? How do we present a quantitative measure of their performance?”
Behkam along with Suh and current doctoral student Ying Zhan tested their nanoparticle-attached salmonella in lab-grown tumors. They found up to 80-fold improvements in nanoparticle penetration and distribution using the NanoBEADS platform, compared to passively diffusing nanoparticles.
Furthermore, Suh and Behkam found out that NanoBEADS largely penetrate the tumor by translocating through the space in between cancer cells.
Behkam wanted to strengthen the NanoBEADS results past the in vitro stage. With a top-flight veterinary school down the road, she enlisted Allen, her fellow MII faculty member, to test the NanoBEADS system in vivo. Tests in breast cancer tumors in mice produced results showing significant improvements compared to passive delivery.
The tests showed that there was about 1,000 times more salmonella cells in the tumor compared to the liver and 10,000 times more than the spleen.
“Most notably, the salmonella itself helped keep the particles in the tumor up to 100-fold better, which would suggest it would be an effective delivery vehicle,” Allen said.
The next step in the research is to load cancer therapeutics into the NanoBEADS system to test the potential enhancement in efficacy.
From bench to kennel to bedside
The collaboration highlights the diversity of interdisciplinary research possible through MII and Virginia Tech.
“The synergistic integration of diverse expertise has been essential to the high-impact discoveries that resulted from this work,” Behkam said.
With the addition of the Virginia Tech Carilion School of Medicine and Fralin Biomedical Research Institute at VTC, Allen said Virginia Tech has the possibility to test scientific research “from bench to kennel to bedside.”
“The project could not move forward without each of the three parts,” Allen said. “The study would not have gotten into such a high impact journal without having the chemistry, the background of the pathogen, the idea, and having the physiological and clinical relevance of testing it in an actual tumor in an actual animal model.”
Davis said all drug delivery mechanisms have to go through animal trials, so having an “absolutely fantastic” college of veterinary medicine on campus took the research to a higher level.
“One thing that attracted me to this project was the ability to work with people like Bahareh and Coy who work with cells and animal studies to really translate the work,” Davis said. “It’s hard to find that combination of people in a lot of schools.”
文章來自: PHYS.ORG (Eng)