抗癌新招 激光「燒斷」癌細胞偽足阻其轉移
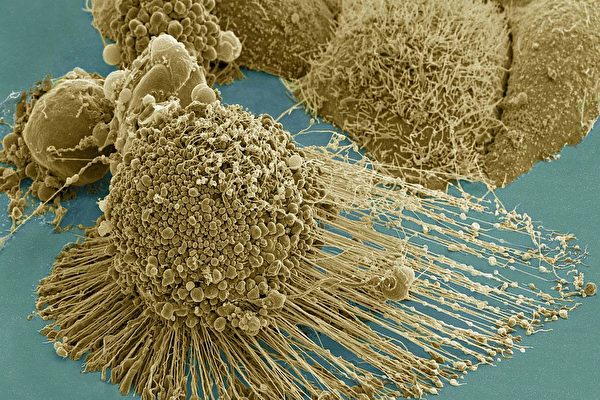
電子掃瞄顯微鏡下拍攝到的癌細胞,長有大量的絲狀偽足,使它們能在人體內轉移。(NIH)
【大紀元2018年01月15日訊】(大紀元記者張妮編譯報導)癌症研究領域頂尖的一批科學家們正在研究一種利用納米「金棒」注入患處,並結合激光熱療的治癌方案,不僅能完成局部治療而不傷害健康細胞,還能有效阻斷癌細胞轉移。該研究已在老鼠體內、以及人體癌細胞實驗室培養皿試驗都獲成功,等待進一步臨床人體試驗。
很多癌症病人在接受治療後,都是被可惡的癌症轉移奪走了生命。因此科學家在研究,如果能找到有效的阻止癌細胞轉移的方式,就會極大地提高癌症的治癒率。
美國國家科學院院士Mostafa El-Sayed引領來自佐治亞理工學院(Gatech)、佐治亞州立大學的科學家們進行的,近期發佈在《美國國家科學院院刊》(PNAS)上的報告揭示了他們的成果進展。
治療原理
研究者們解釋說,如果把人體細胞放大來看,它們就像一個個由薄膜包裹著的充滿著液體的球體,裡面漂浮著細胞器。細胞內還有支撐細胞形態、提供各種功能的細胞骨架。而這些支架帶有許多稱為絲狀偽足的剛毛突起,從細胞壁上延伸出來,幫助正常細胞在它們所屬的人體器官上移動。
但在癌變的細胞上,這些部份通常處於過渡生長狀態,比如會長出大量的絲狀偽足。「這些板狀偽足和絲狀偽足給了癌細胞用於移動的『腿』,使它們能在人體內轉移。」研究者之一、生化分析系畢業生Yue Wu說。
該團隊研究的治療方案,就是向癌變患處注入納米「金棒」。所謂納米金棒,就是細到只有數十億分之一米的黃金材料,納米就是其體積極其微小的意思。這種納米材料分兩步來對付癌細胞。
第一步,將這些材料注入患處讓它們「裹住」癌細胞。這種材料表面附有RGD肽,使其更易與一種稱為整聯蛋白的細胞蛋白質結合。
「這些納米棒綁定整聯蛋白,阻斷它們的功能。這樣它們就無法引導細胞骨架不斷生長板狀和絲狀偽足。」另一位研究者、數據信息生物學博士後助理Yan Tang說。
這就減緩了癌細胞的繁殖速度,而且該過程不會影響正常的細胞。「(因為)某些特定的整聯蛋白只在癌細胞上大量存在,而正常細胞上並不多見。」該研究的主要作者之一Moustafa Ali說。
激光微熱治療
第二步,研究者們用近紅外光(NIR)照射患處,這一療程明顯地停止了癌細胞的轉移。
「細胞不會吸收這種光,但是納米黃金材料會吸收,吸收後會升溫,繼而熔化它們綁定的癌細胞,摧毀板狀和絲狀偽足。」Ali說,「在這次試驗中我們並沒有殺死癌細胞。因為那樣我們就看不到這個方法是否會阻止癌細胞的轉移了。如果需要,我們可以調整療程為殺死癌細胞的模式。」
前一個在動物體內進行的試驗中效果不大好,使用的溫度太高了,「導致發炎,這樣就只能進行一個療程了。」Ali說,「結果雖然大部份癌細胞被殺死了,但是還有少數活下來了,後期還會轉移。」
「而新採用的較溫和的療法不會傷害皮膚或機體組織,這樣就能進行多個療程,能更徹底地阻斷癌細胞遷移的能力。」研究員Ronghu Wu說。
研究者們預計該療法將來適用於頭、頸、乳腺和皮膚癌,能夠局部注入納米金材料,再加以激光低熱熱療,而不像化療讓整個機體都很受傷。
這樣低熱激光大約能擊中埋入組織中2至3厘米深的納米金材料,Ali說「最深能擊入4至5厘米的患處吧」。對於更深部位的腫瘤,「可能需要光纖或內鏡激光設備。」 El-Sayed說。
而將納米材料直接注入血液中目前來看還不可行。
該研究團隊之前在PNAS上發表了成功治療老鼠的研究論文。接受治療的老鼠在15個月後,沒有發現任何對納米金材料的毒性反應。「大部份去了肝臟和脾臟,但這些器官仍正常工作,老鼠一年多後仍健康存活。」
責任編輯:朱涵儒
Thwarting Metastasis by Breaking Cancer’s Legs with Gold Rods
By Ben Brumfield | June 26, 2017 • Atlanta, GA
.jpg)
A dying cancer cell with filopodia stretched out to its right. The protrusions help cancer migrate. Stock NIH NCMIR image. The image does not display a cell treated in the Georgia Tech study. Credit: NIH-funded image of HeLa cell / National Center for Microscopy and Imaging Research / Thomas Deerinck / Mark Ellisman. Use may require permission.
“Your cancer has metastasized. I’m sorry,” is something no one wants to hear a doctor say.
Cancer cells kill most often by crawling away from their original tumors to later re-root in vital parts of the body in a process called metastasis. Now, a research team led by the Georgia Institute of Technology has developed a new treatment to thwart cancer's spread through the body by, in a sense, breaking cancer cells’ legs.
Cancer cells often cover themselves with bristly leg-like protrusions that enable them to creep. The researchers have used minuscule gold rods heated gently by a laser to mangle the protrusions, according to a new study. The treatment prevented cell migration, a key mechanism in metastasis, in experiments on common laboratory cultures (in vitro) of cancerous human cells.
The method could potentially, in the future, offer clinicians going after individual tumors a weapon to combat cancer’s deadly spread at the same time. The medical field is currently less than well-equipped to stop metastasis.
“If cancer stays in a tumor in one place, you can get to it, and it’s not so likely to kill the patient, but when it spreads around the body, that’s what really makes it deadly,” said lead researcher Mostafa El-Sayed, Julius Brown Chair and Regents Professor at Georgia Tech’s School of Chemistry and Biochemistry.
The treatment can also easily kill cancer cells, but in this experiment, it was vital to specifically show that it greatly slowed cell migration. The method is not scheduled for human testing.
Halting cancer softly
The experimental treatment also spared healthy cells, in these and in prior experiments, making the method potentially much less taxing on patients than commonly used chemotherapy. In past tests in animal models, the researchers have uncovered no toxic side effects from the gold used in the treatment, and have found no observable damage to healthy tissue from the low-energy laser.
And they did not see recurrence of the treated cancer.
“The method appears to be very effective as a locally administered treatment that also protects the body from cancer’s spread away from the treated tumors, and it is also very mild, so it can be applied many times over if needed,” El-Sayed said.
El-Sayed, co-lead author Ronghu Wu, and first authors Yue Wu and Moustafa Ali published the results of their current in vitro experiments, a new development in photothermal gold nanorod therapy, on June 26, 2017, in the Proceedings of the National Academy of Sciences. The research was funded by the National Science Foundation and the National Institutes of Health.
How it works: Icky legs
To understand how the treatment works, let’s take a close-up look at a cell and some things that happen to it in malignant cancer.
Many people think of cells as watery balloons — fluid encased in a membrane sheath with organelles floating around inside. But that picture is incomplete. Cells have support grids called cytoskeletons that give them form and that have functions.
The cytoskeletons also form bristly protrusions called filopodia, which extend out from a weave of fibers called lamellipodia that are on the cell’s fringes. The protrusions normally help healthy cells shift their location in the tissue that they are part of.
But in malignant cancer, normally healthy cell functions often lunge into destructive overdrive. Lamellipodia and filopodia are wildly overproduced.
“All these lamellipodia and filopodia give the cancer cells legs,” said Yue Wu, a graduate student in bioanalytical chemistry. “The metastasis requires those protrusions, so the cells can travel.”
How it works: Sticky rods
The gold nanorods thwart the protrusions in two ways. The rods are comprised of a small collection of gold atoms – nano refers to something being just billionths of meters (or feet) in size.
First, El-Sayed’s nanorods are introduced locally, where they encumber the leggy protrusions on cancerous cells. The rods are coated with molecules (RGD-peptides) that make them stick specifically to a type of cell protein called integrin.
“The targeted nanorods tied up the integrin and blocked its functions, so it could not keep guiding the cytoskeleton to overproduce lamellipodia and filopodia,” said Yan Tang, a postdoctoral assistant in computational biology who worked on the study. The binding of the integrin alone slowed down the migration of malignant cells.
But healthy cells were not targeted. “There are certain, specific integrins that are overproduced in cancerous cells,” said Moustafa Ali, one of the study’s first authors. “And you don’t find them so much in healthy cells.”
How it works: Gentle laser heating
In the second phase, researchers hit the gold nanoparticles with a low-energy laser of near-infrared (NIR) light. It brought the migration of the cancer cells to an observable halt.
“The light was not absorbed by the cells, but the gold nanorods absorbed it, and as a result, they heated up and partially melted cancer cells they are connected with, mangling lamellipodia and filopodia,” Ali said. “It didn’t kill all the cells, not in this experiment. If we killed them, we would not have been able to observe whether we stopped them from migrating or not.”
If desired, the treatment can also be adjusted to kill the cells.
Early experiments in animal models in vivo with hotter lasers didn’t work as well. “That caused inflammation, which made it possible to heat one time only,” Ali said. “As a result, that high temperature would wipe out many cancer cells, but not all of them. Some hidden ones might have survived, and also still been able to migrate.”
“This gentle laser didn’t burn the skin or damage tissue, so it could be dosed multiple times and more thoroughly stop the cancer cells from being able to travel,” said researcher Ronghu Wu.
Medical possibilities
The researchers presently envision treating head, neck, breast, and skin cancers with direct, local nanorod injections combined with the low-power near-infrared laser, which can hit the gold nanorods 2-3 centimeters (a bit under or over an inch) deep inside tissue. “But it could go as deep as 4-5 centimeters,” Ali said.
Deeper tumors could conceivably be treated with deeper injections of nanorods. “Then you’d need to go in with a fiber optic or endoscopic laser,” El-Sayed said. Injecting the nanorods directly into the bloodstream as a broad treatment would not currently be a viable option.
El-Sayed’s group has previously published in vivo experiments in mice in the Proceedings of the National Academy of Sciences together with Emory University School of Medicine. That study showed no observable toxicity from the gold in mice 15 months after treatment.
“A lot of it ended up in the liver and spleen,” El-Sayed said. “But the functions of these organs appeared intact upon examination, and treated mice were alive and healthy over a year later.”
Presidential honors
Mostafa El-Sayed is one of the world’s most highly decorated and cited living chemists, and a pioneer of nanoscience and technology. Among his many recognitions are the President’s National Medal of Science, awarded by President George W. Bush, and the Priestley Medal, the American Chemical Society’s highest honor. President Barack H. Obama appointed El-Sayed to the President’s National Medal of Science Committee. El-Sayed also participated in the nomination of chemistry Nobel Laureate Ahmed Zewail.
El-Sayed is known throughout physical chemistry for “El-Sayed’s Rule,” which handles complexities of electron spin orbits, and which has found a lasting place in photochemistry textbooks. After losing his wife to cancer in 2005, El-Sayed dedicated his knowledge and research to ending the scourge.
Also read: Cancer and Technology
Also read: Punching Cancer with RNA Knuckles
The following authors also contributed to this research: Haopeng Xiao and Tiegang Han from Georgia Tech, and Kuangcai Chen and Ning Fang from Georgia State University. This research was funded by the National Science Foundation Division of Chemistry (grants 1608801, CAREER Award CHE-1454501), and the National Institutes of Health Nanotechnology Study Section (grant 1R01GM115763). Any opinions, findings and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the funding agencies.
文章來自:大紀元 / Georgia Tech
中 : http://www.epochtimes.com/b5/18/1/15/n10058064.htm
Eng : http://www.news.gatech.edu/2017/06/26/thwarting-metastasis-breaking-cancers-legs-gold-rods

















.jpg)


